ChemInform Abstract: Stereospecific Synthesis of trans-Arachidonic Acids.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
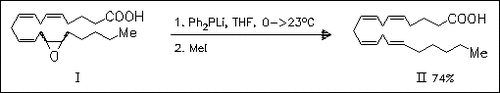
A synthetic procedure to convert cis-arachidonic acids (I) into corresponding trans-isomers (II) applicable on all four double bonds is described. The prepared trans-compounds can be used to investigate the role of trans-arachidonic acids in cardiovascular diseases.