ChemInform Abstract: Practical Stereoselective Synthesis of Conformationally Constrained Unnatural Proline-Based Amino Acids and Peptidomimetics.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
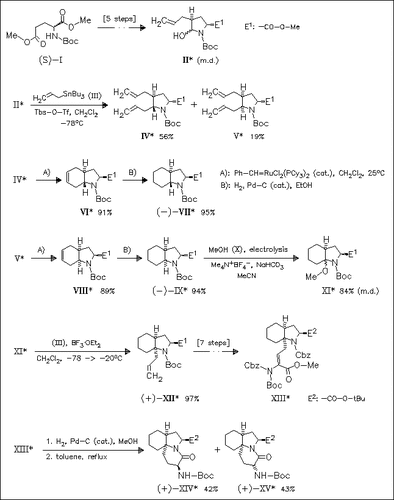
Both trans- and cis-fused esters (VII) and (IX) are prepared by a route involving the allylation of a diastereomeric mixture of alcohol (II) affording trans-diallyl derivative (IV) predominantly, and the ring-closing metathesis of diallylated products (IV) and (V) to give after hydrogenation the target unnatural amino acid esters. Regioselective electrochemical oxidation at the 7a position of the indole nucleus of cis ester (IX) followed by allylation provides derivative (XII) which allows the synthesis of trinuclear dipeptide mimics (XIV) and (XV).