ChemInform Abstract: KF—Al2O3 is an Efficient Solid Support Reagent for the Acetylation of Amines, Alcohols, and Phenols. Impeding Effect of Solvent on the Reaction Rate.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
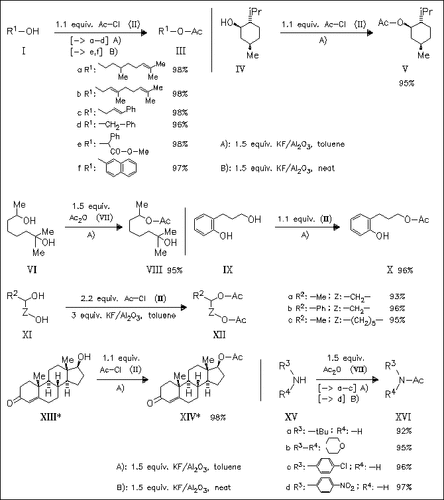
Acetylation of a wide range of alcohols and amines proceeds smoothly with acetyl chloride or acetic anhydride resp., in the presence of Al2O3-supported KF. Primary and secondary alcohols are selectively acetylated in the presence of tertiary or phenolic alcohols [cf. acetylation of diols (VI) and (IX)]. Tertiary alcohols are not acetylated at all. It is shown that in the absence of solvent toluene, the acetylation reaction proceeds faster, which is especially valuable for alcohols and amines not reactive enough for the original acetylation protocol [e.g. β-naphthol (If) or nitroaniline (XVd)].