ChemInform Abstract: Total Synthesis and Stereochemical Revision of (+)-Aeruginosin 298-A.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
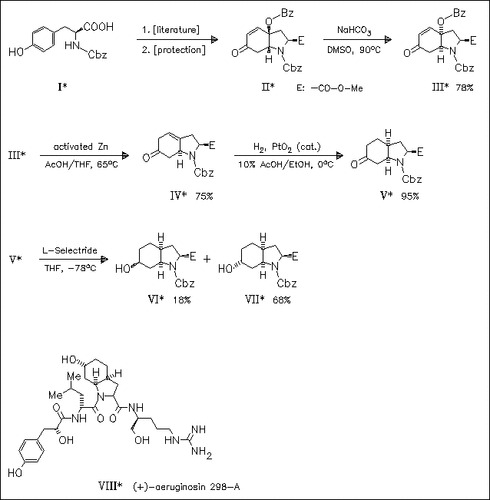
Synthesis of key intermediate (VII) is based on the recently published oxidative cyclization of L-tyrosine. Preparation of aeruginosin incorporating the proposed L-leucine residue is mentioned to fail in providing the natural product. Thus, the structure of title thrombin inhibitor (VIII) is reassigned to contain a D-leucine moiety.