ChemInform Abstract: A Convenient Synthesis of cis-Urocanic Acid, an Endogenous Immunosuppressant.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
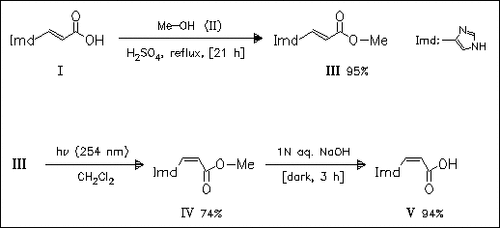
(Z)-Urocanic acid (V) can be prepared conveniently in a preparative scale from naturally occurring (E)-urocanic acid (I) by its conversion to the methyl ester (III), followed by photoisomerization and final hydrolysis. The previously reported direct photoisomerization of acid (I) requires long reaction times and provides no large quantities of isomerized product because of the poor solubility of acid (I) in a majority of solvents.