ChemInform Abstract: General Syntheses of 1-Alkyltoxoflavin and 8-Alkylfervenulin Derivatives of Biological Significance by the Regioselective Alkylation of Reumycin Derivatives and the Rates of Transalkylation from 1-Alkyltoxoflavins into Nucleophiles.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
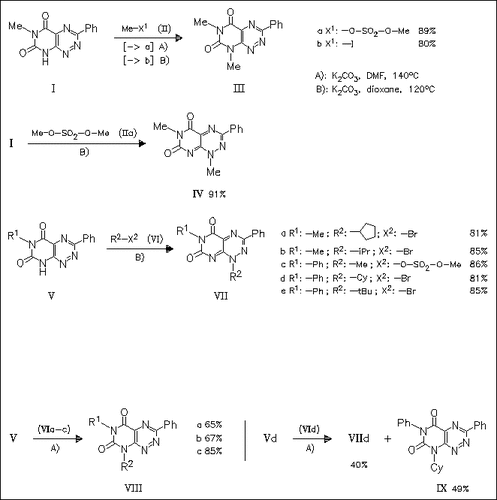
The regioselectivity of N-alkylation of reumycin derivatives (I) and (V) is governed mainly by the nature of the alkylating agent and the solvent used. Thus, alkylation of reumycins with primary alkyl sulfates and secondary or tertiary alkyl bromides (IIa) and (VI) in refluxing dioxane in the presence of K2CO3 cleanly affords 1-alkyltoxoflavin derivatives (IV) and (VII), while in refluxing DMF the 8-alkylfervenulin derivatives (III) and (VIII) are formed in high yields. In contrast to alkyl sulfates [cf. (IIa)], the primary alkyl iodides [cf. (IIb)] afford alkylfervenulins in refluxing dioxane. The transalkylation from 1-alkyltoxoflavins to various nucleophiles, e.g. DMF or butylamine, is studied using model reactions.