ChemInform Abstract: Preparation of Novel Polysubstituted Chiral Cyclohexanone Derivatives Containing a Quaternary Carbon by Ferrier Reaction.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
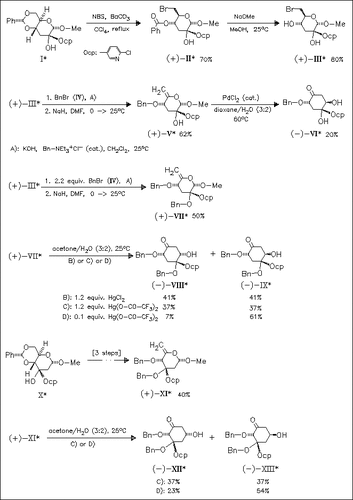
Seven new chiral cyclohexanones are prepared from 2-C- and 3-C-chloropyridyl pyranosides, which are obtained from benzylidene acetals (I) and (X) in three steps, by palladium or mercury salt mediated carbocyclization. The ratio of the α- and β-cyclohexanones is dependent on the nature and concentration of the catalyst. Unsaturated product (V), possessing the quaternary center at C-2, shows no reaction with mercury salts to obtain carbocyclic products, regardless of the conditions