ChemInform Abstract: Anomalous Zemplen Deacylation Reactions of α- and β-D-Mannopyranoside Derivatives.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
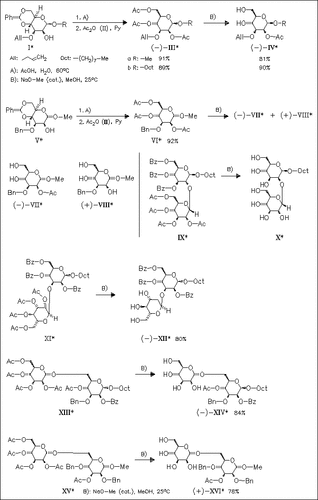
Mono-, di-, and trisaccharides of methyl β-D- and octyl β-D-mannopyranosides having ester groups at isolated and non-isolated positions are partially deacylated to give derivatives in which the O-acyl groups are retained at isolated sites [cf. (IV)]. Isolated 2-O-acyl groups on methyl α-D-mannopyranosides, e.g. (VI), are more labile than on the corresponding β-mannosides. Surprisingly, in the case of disaccharide (XI), all the benzoyl groups remain at the β-mannosidic unit, while all acetyl functions are removed from the nonreducing end. Zemplen reaction of (XIII) gives a product in which not only the ester group at position 2 but the group at position 4 is retained at the reducing end. At non-isolated positions, acyl migration may take place, thus transferring acyl groups to less hindered positions.