ChemInform Abstract: A New Route to Hydrophobic Amino Acids Using Copper-Promoted Reactions of Serine-Derived Organozinc Reagents.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
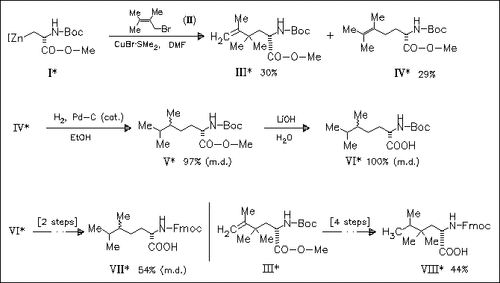
In continuation of earlier works, 6 novel hydrophobic amino acid derivatives such as (VII) and (VIII) are synthesized. The key step in this approach is the Cu-catalyzed reaction of the serine-derived zinc reagent (I) with different allyl electrophiles.