ChemInform Abstract: Chiral Auxiliaries for Asymmetric Radical Cyclization Reactions: Application to the Enantioselective Synthesis of (+)-Triptocallol.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
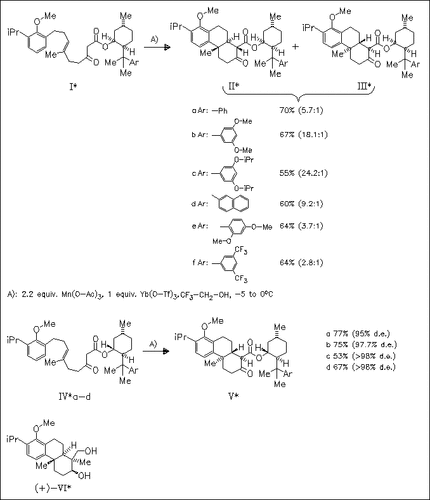
The usefulness of a series of 8-arylmenthyl groups as chiral auxiliaries in the manganese-promoted asymmetric radical cyclization reaction of β-keto esters (I) and (IV) is studied. The best asymmetric induction is observed for auxiliaries containing electron-donating substituents at the meta-positions of the aryl ring [cf. (Ic) and (IVc)]. Cyclization of epimeric β-keto esters (I) and (IV) provides access to both enantiomers of the target tricyclic skeleton. Finally, compound (IIc) is used to generate triptocallol (VI) in 90% optical purity.