ChemInform Abstract: Transformations of 1,2,4-Triazines in Reactions with Nucleophiles. Part 4. Nucleophilic Substitution of Hydrogen in 1,2,4-Triazine 4-Oxides under Acylation Conditions.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
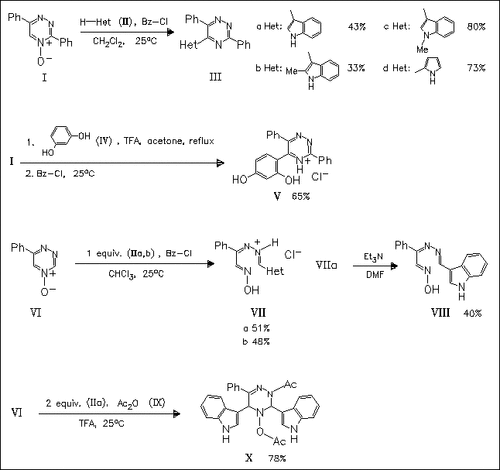
Reaction of the 3-substituted triazine oxides (I) with (het)aromatic nucleophiles in the presence of benzoyl chloride affords the corresponding 5-(het)aryl-substituted analogues (III) and (V). The 3,5-unsubstituted triazine oxide (VI), however, shows a different regioselectivity of nucleophilic attack under analogous conditions and provides triazahexatrienes (VII) by triazine ring opening. In contrast, in the presence of acetic anhydride disubstitution of the triazine ring is observed to give (X). The reaction mechanisms are studied in detail and several possible intermediates are identified.