ChemInform Abstract: Formation of 1,2-Oxazine Derivatives: A Novel Type of Reaction Between 2-Aminooxazoles and Maleic Acid Imide.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
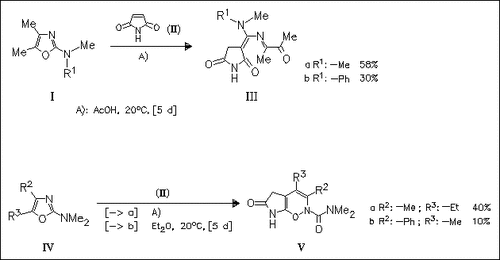
Reaction of N,N-disubstituted 2-aminooxazoles with maleimide (II) results in the formation of alkylidenesuccinimides (III) or pyrrolooxazines (V) depending on the substitution pattern of starting oxazoles. The formation of the fused heterocycles (V) is explained by an unprecedented C5—O bond cleavage of oxazoles (IV) followed by ring closure of the intramolecular diene synthesis type.