ChemInform Abstract: Totally Hindered Phenols. 2,6-Di-t-butyl-4-(1,1-dialkyl-1-acetamide)-phenols and Their Persistent Phenoxy Radicals.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
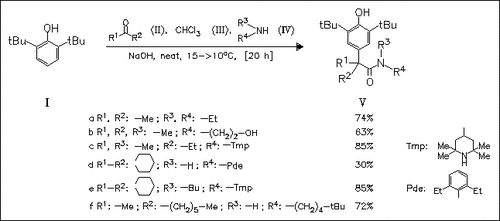
Condensation of 2,6-di-tert-butylphenol with ketones, chloroform, and amines is effected by solid NaOH yielding amides of type (V). Their phenoxy radicals are found to be more persistent than the known 2,4,6-tri-tert-butylphenoxy radical.