ChemInform Abstract: Stereoselective Synthesis of Secondary Organozinc Reagents and Their Reaction with Heteroatomic Electrophiles.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
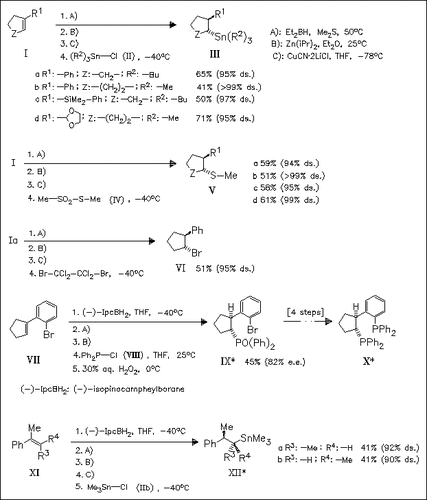
Cyclic and acyclic trisubstituted alkenes are transformed to stable trans-configured diorganozinc reagents, which smoothly undergo reaction with various heteroatomic electrophiles to furnish tin-, sulfur-, or bromine-containing adducts in moderate yields and with retention of configuration. The utility of the method is demonstrated in the preparation of a chiral bisphosphine ligand (X) by enantioselective introduction of a phosphine oxide substituent into cyclic olefin (VII).