ChemInform Abstract: Highly Stereoselective Intramolecular Michael Addition Using α-Sulfinyl Vinyllithium as an Unprecedented Michael Donor.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
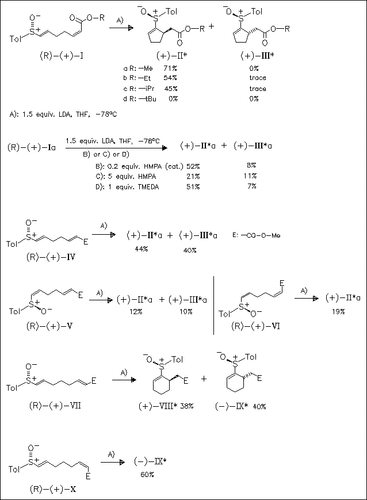
From the examination of the four geometric isomers (Ia) and (IV)-(VI) in the title reaction it is found, that (Z)-enoates (I) and (VI) give cyclopentene sulfoxides (II) with excellent diastereoselectivity and (R)-configuration of the newly formed stereogenic center. (Z)-Enoate (X) affords cyclohexene (IX) also with excellent diastereoselectivity, but with (S)-configuration of the newly formed stereogenic center. (E)-Enoates provide the cycloadducts only with poor diastereoselectivity.