ChemInform Abstract: Medium-Sized Cyclophanes. Part 53. Synthesis and Conformational Studies, and Photoinduced Cyclization of syn-[n.2]Metacyclophanenes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
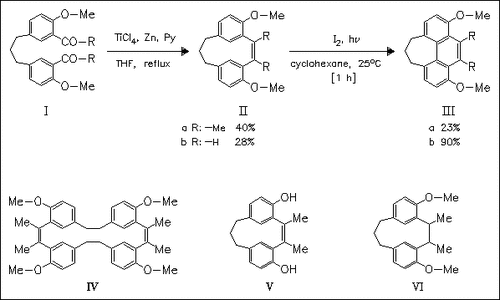
McMurry cyclization of bis(acetylphenyl)alkanes (I) provides access to the title metacyclophanenes (II). Compound (IIb) exists in the expected anti-conformation, while compound (IIa) possesses syn-conformation due to the influence of substituents at the double bond. Interestingly, the lower homologue of compound (Ia) affords only the dimer (IV) during cyclization due to steric strain. Irradiation of metacyclophanenes (II) in the presence of iodine proceeds with cyclization to give phenanthrene derivatives (III). It is shown that anti-(IIb) reacts much faster than syn-(IIa). The syn/anti-conformation is studied also in some derivatives of metacyclophanene (IIa), namely compounds (V) and (VI).