ChemInform Abstract: Asymmetric Reduction of Ethynyl Ketones and Ethynylketoesters by Secondary Alcohol Dehydrogenase from Thermoanaerobacter ethanolicus.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
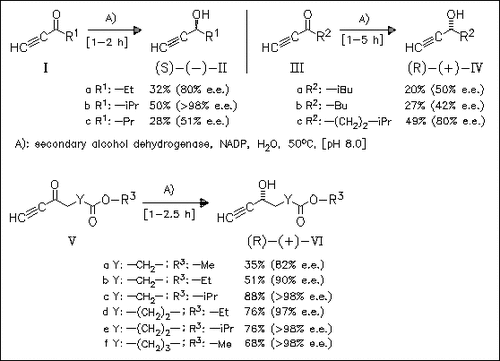
Ethynyl ketones are reduced with moderate enantioselectivities, except for isopropyl derivative (Ib), by the title oxidoreductase to afford (S)-alcohols from ketones (I) bearing small alkyl substituents and (R)-alcohols from ketones (III) bearing larger substituents. Whereas secondary carbons are tolerated in substituents, tertiary alkyl groups in the substrates result in very slow reactions and marginal yields. In contrast, esters (V) are converted to (R)-hydroxyesters (VI) with excellent optical purity.