ChemInform Abstract: Asymmetric and Nonasymmetric Addition of RLi and RMgX to 3-Methoxynaphthalen-2-yl Oxazolines and Imines. An Approach to Substituted 2-Tetralones.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
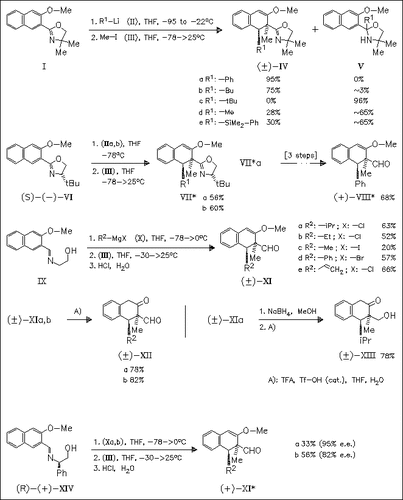
It is shown that the 3-methoxynaphthalen-2-yl oxazolines (I) and (VI) do not undergo aromatic nucleophilic substitution of the 3-MeO group with organolithium reagents but afford dihydronaphthalenes like (IV) and (VII). The latter can easily be transformed into aldehydes of type (VIII). These aldehydes can also be prepared via addition of Grignard reagents to 3-methoxynaphthalen-2-yl imines. The aldehydes are useful precursors of substituted 2-tetralones. The analogue containing a 2-carbomethoxy group is a highly versatile intermediate from which 4-, 3,4-, and 3,4,4-substituted 2-tetralones are available.