ChemInform Abstract: An Efficient Synthesis of N-Unsubstituted Imines as Organoborane Adducts Stable at Room Temperature: New Promising Intermediates for Synthesis.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
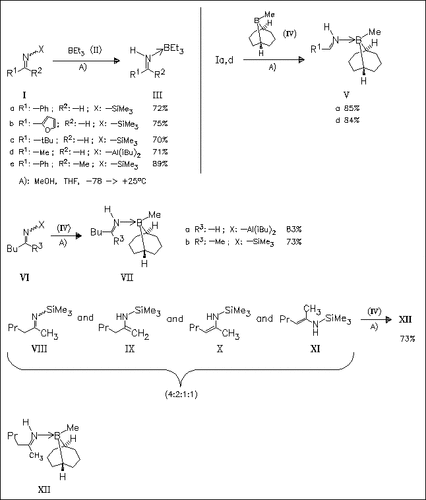
N-Trimethylsilyl- or N-diisobutylaluminoimines are readily methanolyzed in the presence of boranes to afford N-unsubstituted aldimine— as well as ketimine—borane adducts as (E)-isomers exclusively. Methanolysis of the mixture of enamine isomers (VIII)—(XI) in the presence of borane (IV) affords solely the stable ketimine—borane adduct (XII) as result of conversion of isomers (IX)-(XI) to the ketimine form.