ChemInform Abstract: Catalytic Use of Chiral Phosphine Ligands in Asymmetric Pauson—Khand Reactions.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
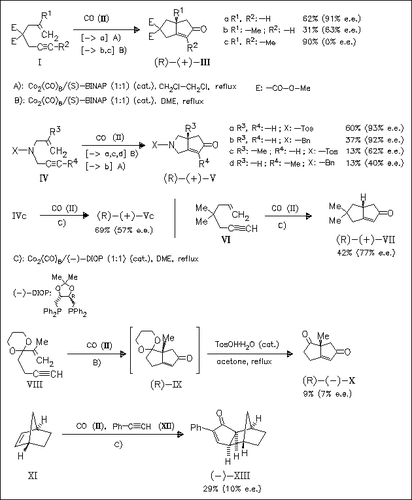
The first example of catalytic asymmetric syntheses of 2-cyclopentenone systems by intramolecular Pauson—Khand reaction of 1,6-enynes using catalytic amounts of Co2(CO)8 and chiral phosphine ligand are reported. From several tested ligands, (S)-BINAP is found to be the most effective. The presence of a methyl group in the alkynyl or alkenyl part results in a remarkable decrease of the enantiocontrol of the reaction, whereas 1,6-enynes (Ia) and (IVa) without methyl groups provide excellent enantioselectivities. The enantioselectivities obtained in the reaction of ketal (VIII) or in the intermolecular reaction of norbornene with phenylacetylene are only low (<10%).