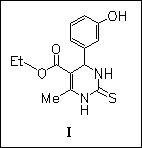
ChemInform Abstract: Synthesis and Reactions of Biginelli Compounds. Part 19. X-Ray Structure, Conformational Analysis, Enantioseparation, and Determination of Absolute Configuration of the Mitotic Kinesin Eg5 Inhibitor Monastrol.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
Computational, X-ray diffraction, and NMR studies show that monastrol (I) is a conformationally mobile heterocyclic system with no clear preference for one of the four distinct conformers. Resolution of the racemate is achieved by direct enantioselective HPLC. The absolute configuration of the first eluting (S)-(+)-enantiomer is established by CD spectroscopy.