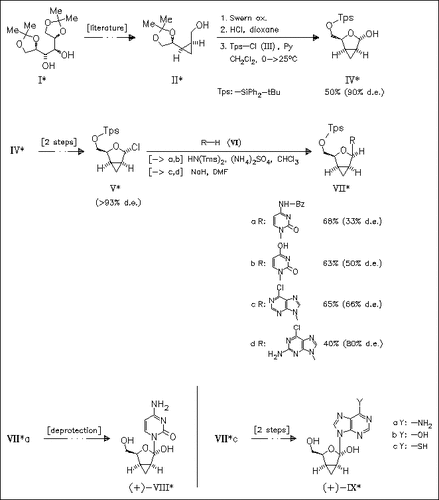
ChemInform Abstract: Enantiomeric Syntheses of Conformationally Restricted D- and L-2′,3′-Dideoxy-2′,3′-endo-methylene Nucleosides from Carbohydrate Chiral Templates.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
Novel nucleosides like (VIII) and (IX) as well as their enantiomers are prepared in order to test their biological activity. None of them shows significant anti HIV-activity.