ChemInform Abstract: Tandem Silylformylation/Wittig Olefination of Terminal Alkynes: Stereoselective Synthesis of 2,4-Dienoic Esters.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
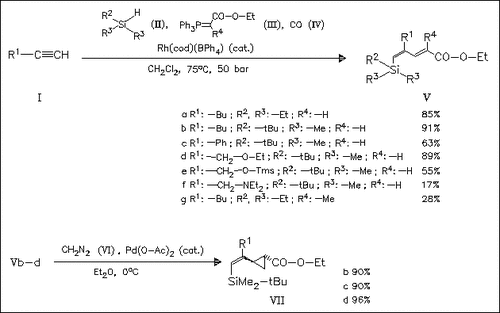
A novel effective and straightforward approach to the synthesis of δ-silylated α,β,γ,δ-unsaturated esters (V) is presented, involving the one-pot tandem silylformylation—Wittig olefination reaction of terminal alkynes (I) with hydrosilanes (II), phosphonium ylides (III) and carbon monoxide (IV) in the presence of a cationic rhodium catalyst. The diene esters (V) are obtained in excellent stereoselectivity. The synthetic utility of these novel diene esters is demonstrated by [2 + 1] cycloaddition of diazomethane. The cyclopropanation takes place at the less substituted double bond and provides the corresponding cyclopropane adducts (VII) in high stereoselectivity.