ChemInform Abstract: Acyclic Analogues of Deoxyadenosine 3′,5′-Bisphosphates as P2Y1 Receptor Antagonists.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
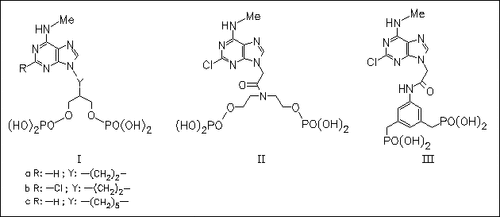
Some new acyclic nucleotide analogues are synthesized as ligands for the P2Y1 receptor. Three groups of adenine derivatives (I), (II), and (III) which contain two symmetrical phosphate or phosphonate groups in place of the ribose moiety are prepared. Their biological activity is demonstrated by the ability to act as agonists or antagonists in the simulation of phospholipase C in turkey erythrocyte membranes. The highest affinity among the three novel structures exhibit the 2-chloro-N-methyl gancyclovir equivalent (Ib).