ChemInform Abstract: Stereoselective Radical-Tandem Reaction of Aniline Derivatives with (5R)-5-Menthyloxy-2,5-dihydrofuran-2-one Initiated by Photochemical Induced Electron Transfer.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
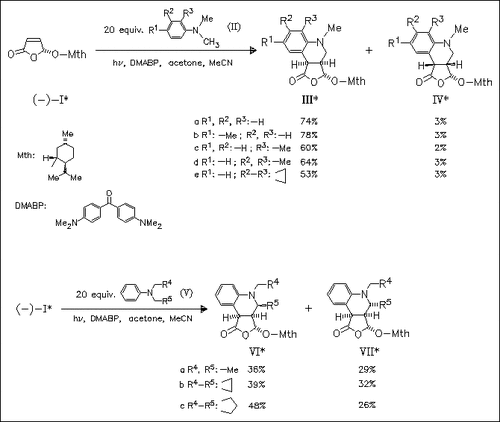
The stereoselective addition of tertiary aromatic amines (II) and (V) to furanone (I) leads to the corresponding quinolines (III), (IV), (VI), and (VII) through a radical-tandem process. Under optimized reaction conditions, the facial diastereoselectivity is about 90%. In the reaction of (I) with (V) the configuration of the second chiral center is not efficiently controlled. Thus, the products (VI) and (VII) are obtained in similar yields.