ChemInform Abstract: Novel and Stereoselective Methods for the Preparation of Aromatic Lactams via Reductive Coupling Reactions Mediated by SmI2.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
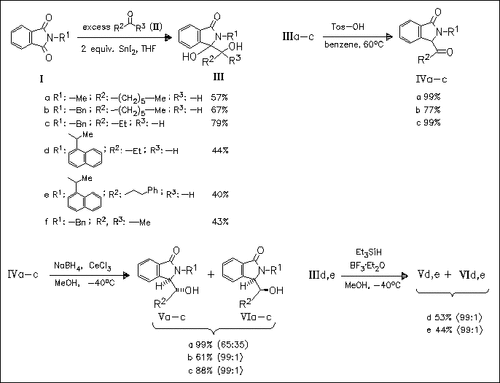
The first example of samarium(II) diiodide mediated coupling reaction of aromatic N-alkylimides (I) with carbonyl compounds (II) is described. The reduction of the corresponding ketoamides (IV), derived from dehydration of the coupling products, with NaBH4 in the presence of CeCl3 occurs with high stereoselectivity, leading to threo-aromatic lactams (V). Furthermore, direct reductive deoxygenation of (III) with Et3SiH in the presence of Lewis acid also affords the same threo-lactams (V) with high stereoselectivity.