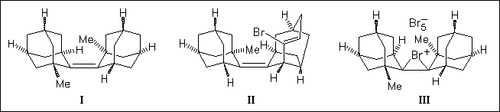
ChemInform Abstract: Steric Strain and Reactivity: Electrophilic Bromination of trans-(1-Methyl-2-adamantylidene)-1-methyladamantane.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
UV-VIS and NMR spectroscopic investigation of the title compound (I)—bromine system in comparison with that of the unsubstituted parent compound, adamantylideneadamantane, shows a marked effect of the steric strain present in (I) on the reactivity of the double bond. At very low bromine concentration, compound (I) is rapidly transformed into rearranged substitution product (II), whereas at high bromine concentration, compound (I) generates a bromonium polybromide ion pair (III), which is stable and unable to further evolve into products.