ChemInform Abstract: Cyclizations of Substituted Benzylidene-3-alkenylamines: Synthesis of the Tricyclic Core of the Martinellines.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
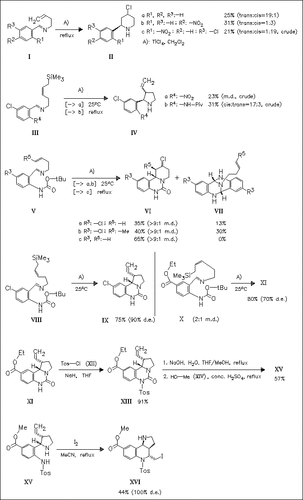
Lewis acid-mediated cyclization of benzylidene-3-alkenylamines leads to different heterocycles (piperidines, pyrrolidines, dibenzodiazocines, pyridoquinazolines, pyrroloquinazolines) depending on the educt structure. Pyrroloquinazolinones such as (IX) and (XI) can smoothly be converted into pyrroloquinolines like (XVI), which are potent intermediates of martinellines.