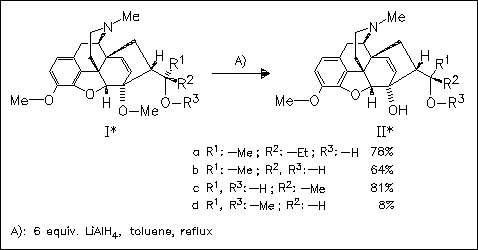
ChemInform Abstract: 6-O-Demethylation of the Thevinols with Lithium Aluminum Hydride: Selective Demethylation of a Tertiary Alkyl Methyl Ether in the Presence of an Aryl Methyl Ether.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
Regioselective demethylation of the 6-O-Me group of thevinol derivatives (I) proceeds smoothly in the presence of excess LiAlH4 in the non-coordinating solvent toluene. It is proposed that a chelating hydroxy or amino group at the C-20 side chain is necessary to give good results in hydrogenolysis, since the methyloxy derivative (Id) provides the demethylation product in low yield.