ChemInform Abstract: Axially Chiral Amidinium Ions as Inducers of Enantioselectivity in Diels—Alder Reactions.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
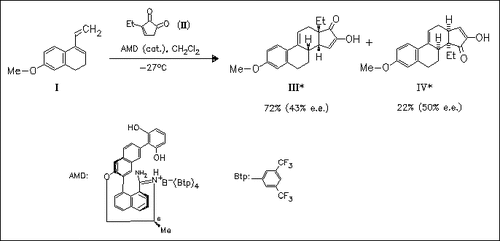
Diketones of type (II) form hydrogen bonds with lipophilic amidinium ions. Thus, the Diels—Alder reaction of diene (I) with dienophile (II) in the presence of catalytic amounts of chiral amidinium salt provides the steroid skeletons (III) and (IV) with moderate enantioselectivities.