ChemInform Abstract: Additional Data on the Synthesis and Properties of Chiral 1,2-Bis(phosphetano)benzenes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
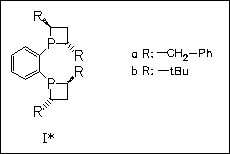
The synthesis of several novel chiral bis(phosphetano)benzenes (I) on a previously described pathway is attempted. However, only the benzyl derivative (Ia) can be synthesized, while the highly bulky tert-butyl analogue is not obtained under the reaction conditions. Screening of the catalytic activity of a ruthenium complex, generated from Ru(cod)(2-methylallyl)2, ligand (Ia), and HBr, in the enantioselective hydrogenation of α-keto esters reveals a comparable activity of the novel ligand to previously described isopropyl or cyclohexyl analogues. The complexation behavior of the novel ligand to palladium and ruthenium is studied.