ChemInform Abstract: Organophosphorus Compounds. Part 144. A Novel Approach to 1,3-Oxaphospholes from Phosphaalkynes and Isomuenchnones.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
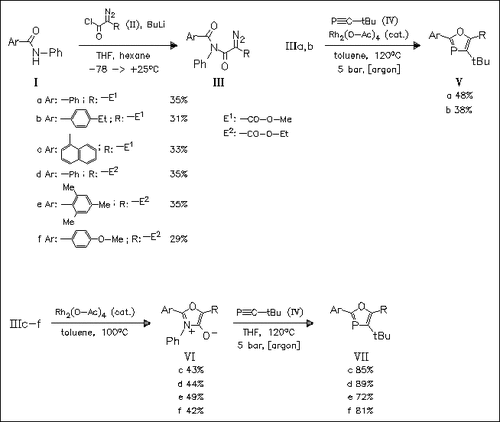
A novel facile and regioselective access to 1,3-oxaphosphole derivatives is presented. Cycloaddition of isomuenchnones (VI), obtained by rhodium-catalyzed cyclization of diazooxopropanoates (III), to phosphaalkynes (IV) regiospecifically provides bicyclic intermediates, which cannot be isolated and immediately decompose via a retro Diels—Alder reaction to provide oxophospholes (VII). This reaction sequence can also be carried out in one step without the isolation of isomuenchnones [cf. (III)→(V)].