ChemInform Abstract: Reactions of (1-Diazo-2-oxoalkyl)silanes with 3H-1,2,3,4-Triazaphospholes: A Route to Short-Lived 4-Imino-1,2,4(λ5)-diazaphospholes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
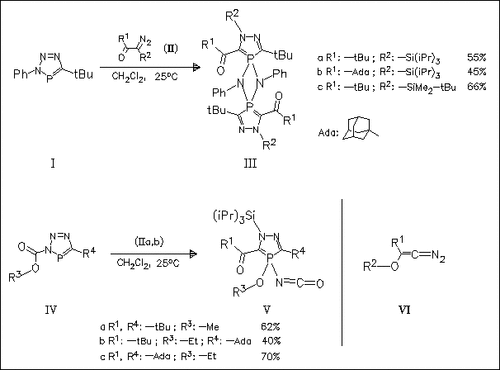
Reaction of diazooxoalkylsilanes (II), which are in equilibrium to diazosilyloxyalkenes (VI), with N-phenyltriazaphospholes (I) starts with [3 + 2] cycloaddition to give a bicyclic dihydrotriazaphosphole adduct, which eliminates nitrogen to afford the title iminodiazaphosphole intermediate. Cyclodimerization of this imine then gives the diazadiphosphetidine derivatives (III). In contrast, the analogous iminodiazaphosphole intermediate, obtained from diazooxoalkylsilanes (II) and N-alkoxycarbonyltriazaphospholes (IV), undergoes 1,3(C→P)alkoxide migration to furnish diazaphospholes (V) in moderate yields.