ChemInform Abstract: Palladium-Catalyzed Borylation of Aryl Halides or Triflates with Dialkoxyborane: A Novel and Facile Synthetic Route to Arylboronates.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
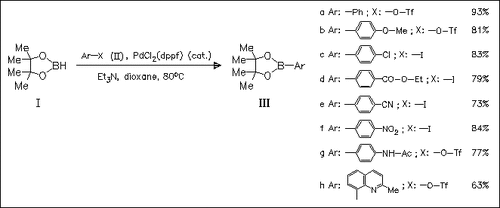
The previously reported title method is successfully extended to aryl sulfonates and thus represents a new route to pinacolboronates of type (III) (17 examples). Generally, aryl iodides exhibit higher reactivity than aryl bromides or sulfonates, but the use of sulfonates has some advantages, in part due to the easy availability from phenols. The present method tolerates various functional groups such as CO2Et, COMe, CN, and NHAc.