ChemInform Abstract: Lewis Acid Mediated Elimination and Rearrangement Reactions of α-Chlorosulfides Derived from Phenylthio-Substituted 4,5-Dihydrofuran-3(2H)-ones.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
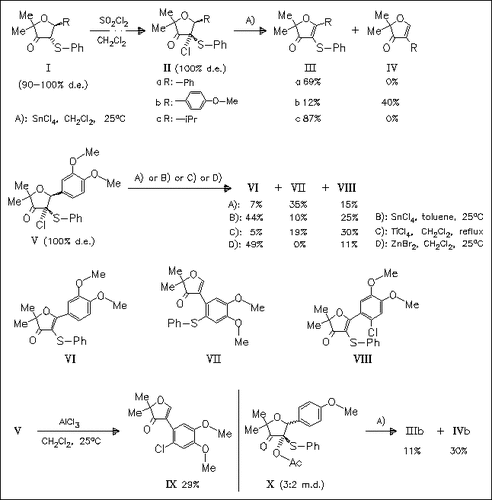
Chloro(phenylthio)dihydrofurans such as (II) and (V), obtained as single diastereomers by treatment of the corresponding phenylthiodihydrofurans with SO2Cl2, undergo elimination and in the appropriate cases aryl group migration reactions by treatment with stoichiometric amounts of Lewis acids. A similar behavior is observed with acetoxy derivative (X). Surprisingly, chlorinated product (VIII) rather than its bromo analogue is formed by treatment of compound (V) with ZnBr2.