ChemInform Abstract: β-Lactams from D-Erythrose-Derived Imines: A Convenient Synthesis of 2,3-Diamino-2,3-dideoxy-D-mannonic Acid Derivatives.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
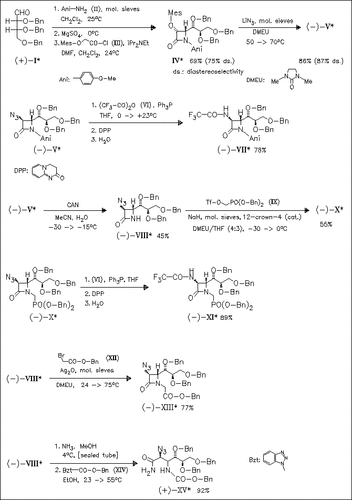
Key step in the title synthesis is the cycloaddition of imines, in situ generated from D-erythrose derivatives like (I) and aniline derivative (II), with the ketene derived from mesyloxyacetyl chloride (III). Transformation of the so obtained mesyloxy-substituted β-lactam (IV) to the azide (V) provides access to a number of further modifications, e.g. formation of the trifluoroacetylamino derivative (VII) or N-dearylation to compound (VIII). Aminolysis and carbamoylation of derivative (VIII) affords the hitherto unknown diamino-dideoxy-D-mannonamide (XV). None of the newly synthesized compounds, obtained by debenzylation of compounds (VII), (XI), and (XIII), possesses inhibitory activity against sialidases isolated from various viruses and bacteria.