ChemInform Abstract: Syntheses of Derivatives of L-Daunosamine and Its C-3 Epimer Employing as the Key Step the Asymmetric Conjugate Addition of a Homochiral Lithium Amide to tert-Butyl (E,E)-Hexa-2,4-dienoate.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
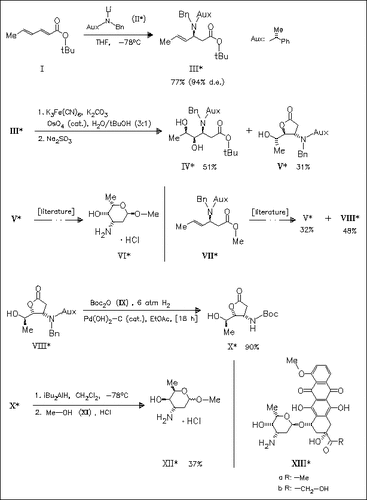
A detailed account of previously published studies on the preparation of methyl α-L-daunosaminide (VI) and methyl-3-epi-D-daunosaminide (XII) is presented. Daunosamine constitutes the glycoside part of the anthracycline antibiotics daunorubicin and adriamycin (XIII).