ChemInform Abstract: Orally Active Antithrombotic Thioglycosides. Part 8. Synthesis of 4-Cyanophenyl and 4-Nitrophenyl 1,5-Dithio-D-ribopyranosides as well as Their 2-Deoxy and 2,3-Dideoxy Derivatives Possessing Antithrombotic Activity.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
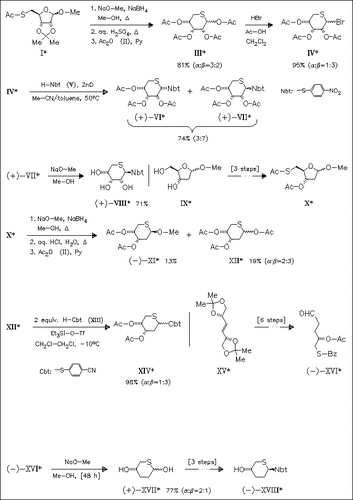
In contrast to a literature statement, the oral antithrombotic effect of 4-cyanophenyl-1,5-dithio-pentopyranosides is not restricted to the β-D-xylo configuration, as previously shown. In order to check the influence of the pentose unit, the corresponding 5-thio-D-ribopyranosides, e.g. (VIII), are synthesized. Furthermore, the presence of the C-2 hydroxyl group is not necessary for the activity. Thus, 2-deoxy and 2,3-dideoxy-ribopyranosides, e.g. (XIV) and (XVIII), are also prepared. With exception of one compound, all pyranosides are more active than the reference compound beciparcil.