ChemInform Abstract: Enantioselective Preparation of γ-Amino Acids and γ-Lactams from Nitro Olefins and Carboxylic Acids, with the Valine-Derived 4-Isopropyl-5,5-diphenyl-1,3-oxazolidin-2-one as an Auxiliary.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
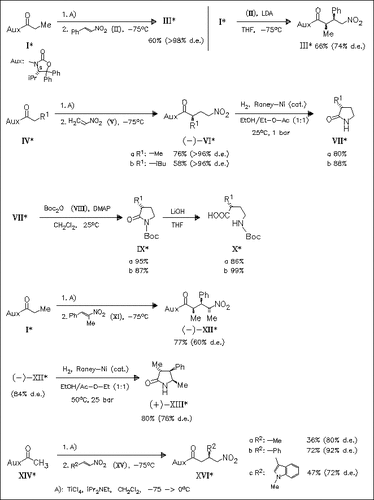
Addition of titanium enolates of N-acyloxazolidinones (I), (IV), and (XIV) to nitroolefins (II), (V), and (XV), resp., in the presence of TiCl4 provides the corresponding nitrocarboxylic amides (III), (VI), and (XVI), resp., in moderate yields and good diastereoselectivity. Hydrogenation of these nitrocarboxylic amides, as described for derivatives (VI), proceeds with cleavage of the chiral auxiliary and subsequent lactamization to afford γ-lactams (VII), which undergo N-protection and lactam ring opening to the N-Boc-protected γ-amino acids [cf. (X)]. Depending on the structure of enolates and nitroolefins, γ-lactams with up to 3 stereocenters [cf. (XIII)] can be prepared. The structure of several nitrocarboxylic amides, e.g. (XVIa), and of lactam (XIII) is studied using X-ray crystallography.