ChemInform Abstract: Diastereoselective Synthesis of β2-Amino Acids.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
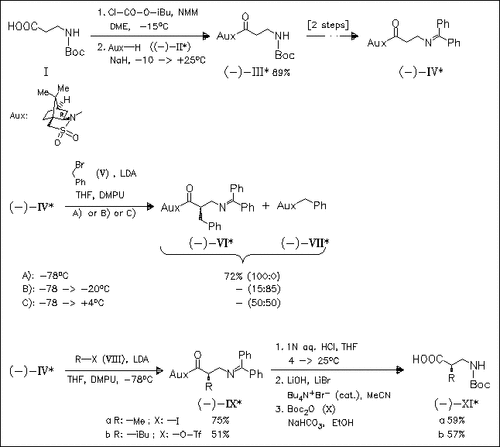
A general route to β2-amino acids is presented, involving highly diastereoselective alkylation of a chiral sultam β-alaninate derivative (IV) as the key step. Alkylation must be performed below -45 °C to avoid elimination via ketene formation. β2-Homophenylalanine (VI), -homoalanine (IXa) and -homoleucine derivatives (IXb) are prepared using this technique. Starting with (-)-sultam (II), the (R)-β2-amino acids are obtained, while the corresponding (S)-enantiomers arise from (+)-sultam.