ChemInform Abstract: Stereodivergent Synthesis of Highly Substituted Tetrahydropyrans.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
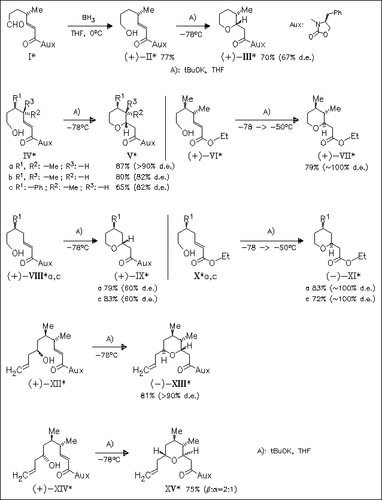
Key step in the stereoselective synthesis of polyalkyl-substituted tetrahydropyrans is the intramolecular oxa-conjugate Michael addition in hydroxyenimides or hydroxyenoates. Generally, the intramolecular addition in hydroxyenimides [cf. (IV) and (VIII)] proceeds with kinetic control, while for the corresponding hydroxyenoates [cf. (VI) and (X)] the addition is thermodynamically controlled. The diastereoselectivity of tBuOK-mediated addition in hydroxyenimides (II), (IV), (VIII), (XII), and (XIV) is shown to depend strongly on the substitution pattern. While high diastereomeric purity is observed for 3,4-disubstituted hydroxyenimides [cf. (IV) and (XII)], monosubstituted analogues (II) and (VIII) react with lower diastereoselection. In the case of secondary hydroxides (XII) and (XIV), the tetrahydrofurans are formed with 2,6-trans-selectivity.