ChemInform Abstract: Lewis Acid Catalyzed Reactions of Thioketones with 1,2-Epoxycyclohexane and 1,2-Epoxycyclopentane.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
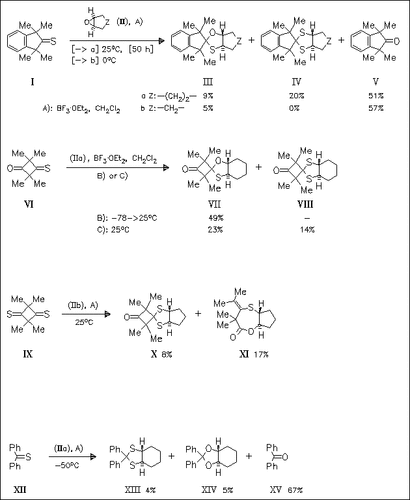
Lewis acid-catalyzed addition of bicyclic oxiranes (II) to various non-enolizable thioketones affords the corresponding cycloalkano-fused 1,3-oxathiolanes as primary adducts. These oxathiolanes are not very stable under the reaction conditions and, in some cases, they can be isolated only at low temperatures. They readily undergo secondary reactions to afford dithiolane or dioxolane analogues as the main products. It is shown that oxathiolane derivatives from epoxycyclopentane (IIb) are less stable as compared to their higher homologues and are mostly not isolated. In all cases, the newly formed bicyclic systems are trans-fused. A plausible reaction mechanism is given, which also explains the rather unexpected formation of fused oxathiepine derivative (XI).