ChemInform Abstract: Ruthenium-Catalyzed Cycloisomerization—Oxidation of Homopropargyl Alcohols. A New Access to γ-Butyrolactones.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
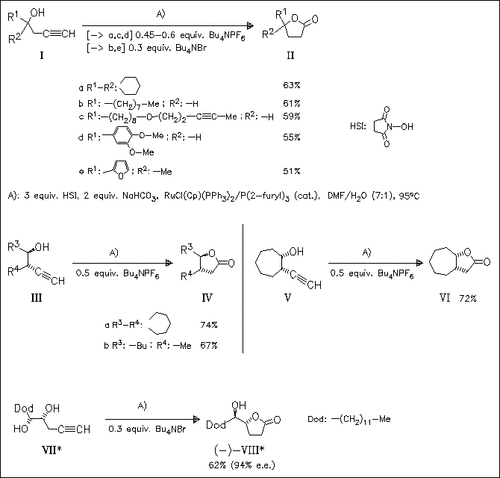
A wide range of homopropargylic alcohols are converted to their corresponding γ-butyrolactones by use of a catalytic amount ruthenium complex/trifurylphosphine, NaHCO3 as a base, N-hydroxysuccinimide as oxidant, and tetrabutylammonium bromide or hexafluorophosphate as additive. A number of the lactones synthesized are naturally occurring compounds. An example for the chemoselectivity of this method is the preparation of muricatacin (VIII) with no formation of the corresponding six-membered lactone.