ChemInform Abstract: Reductive Allylation of Pyrrole with Allylboranes. Synthesis of trans- and cis-2,5-Disubstituted Pyrrolidines.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
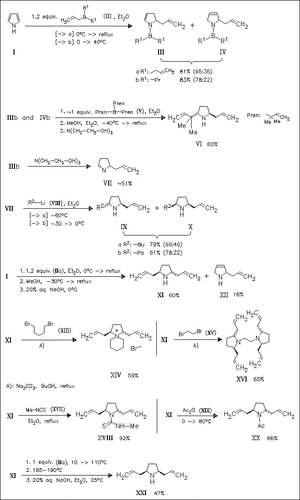
Pyrrole undergoes monoallylation on treatment with β,γ-unsaturated organoboron derivatives to furnish isomeric addition products which differ in position of the double bond in the pyrrole ring [cf. (III), (IV)]. Successive treatment of the monoaddition products with alcohols and bases provides trans-2,5-diallylated pyrrolidines [cf. (VI), (XI)]. Heating the trans-pyrrolidines with triallylborane provides access to the corresponding cis-isomers [→(XXI)]. Moreover, the synthesis of some N-substituted trans-pyrrolidines [cf. (XIV), (XVI), (XVIII), (XX)] and a general procedure for the synthesis of nonsymmetrically substituted trans- and cis-pyrrolidines [cf. (IX) and (X)] based on the reductive allylboration of pyrrole followed by 1,2-addition of appropriate lithium organyls are given.