ChemInform Abstract: New N1-Hetarylmethylene-Substituted Amidrazones with Potential Antimycobacterial Activity.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
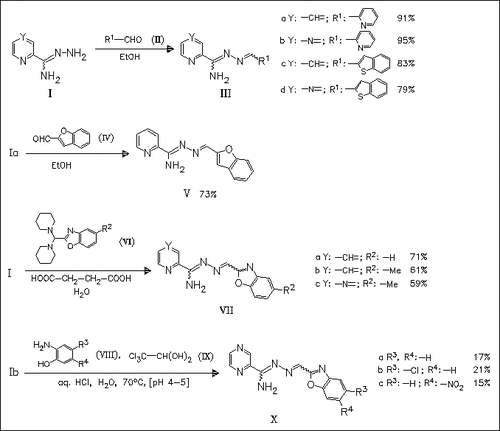
Amidrazones combining the structural moiety of an N1-methylene-substituted amidrazone and some promising hetarylic substituents, which display antimycobacterial activity in previously synthesized amidrazones, are synthesized and tested against various mycobacterial strains. An alternative approach to amidrazones (X) resembles the multistep reaction for 2-benzoxazolylcarbaldoxime. Here, hydroxylamine can be replaced by pyrazinecarboxamidrazone (Ib) to give the amidrazones (X). Amidrazone (V) is obtained as a pure isomer; its structure is investigated using X-ray diffraction studies. None of the new compounds reveals any remarkable antimycobacterial activity.