ChemInform Abstract: Vicarious Nucleophilic Substitution of Hydrogen in 2,4-Dinitroanilines and Cyanonitroanilines.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
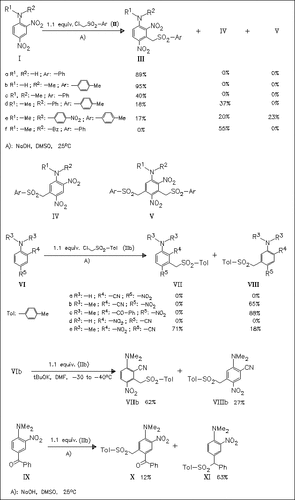
1-Amino-2,4-dinitroanilines (I) prove to be active in vicarious nucleophilic substitution in spite of existing in anionic form under the basic reaction conditions. The regioselectivity of the substitution can be controlled by choice of the amino-substituent. Cyanonitroanilines (VI), although less electrophilic, show similar reactivity features in some cases.