ChemInform Abstract: The SCN- Ion as an Ambidentate Ligand — Synthesis and Crystal Structures of (Bu4N)4 [Ag2Fe2(SCN)12] and (Et4N)21∞ [Ag2Fe(SCN)6].
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
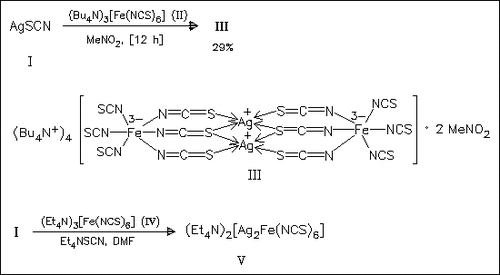
Title compound (III) crystallizes in the triclinic space group P with Z = 1 and (V) is orthorhombic, space group Pnnm (Z = 2). In these compounds ambidentate SCN- anions link Ag+ ions with Fe3+ (III) and Fe2+ (V) ions, respectively.The tetranuclear anion in (III) consists of two [Fe(NCS)6]3- groups connected by two Ag+ ions. The same μ2- and μ3-SCN bridging pattern as in (III) leads to polymeric chains in (V).
with Z = 1 and (V) is orthorhombic, space group Pnnm (Z = 2). In these compounds ambidentate SCN- anions link Ag+ ions with Fe3+ (III) and Fe2+ (V) ions, respectively.The tetranuclear anion in (III) consists of two [Fe(NCS)6]3- groups connected by two Ag+ ions. The same μ2- and μ3-SCN bridging pattern as in (III) leads to polymeric chains in (V).