ChemInform Abstract: Nucleosides and Nucleotides. Part 190. The First Synthesis of Herbicidin B. Stereoselective Construction of the Tricyclic Undecose Moiety by a Conformational Restriction Strategy Using Steric Repulsion Between Adjacent Bulky Silyl Protecting Groups on a Pyranose Ring.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
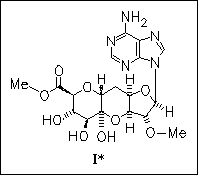
Key step in the first total synthesis of antibiotic herbicidin B (I) is the novel regioselective aldol-type C-glycosidation reaction of methyl 3,4-O-(1,1,3,3-tetraisopropyl-1,3-disiloxanediyl) -1-phenylthio-2-ulos-β-D-glucuronate promoted by samarium diiodide to give the corresponding 1-enolate.