ChemInform Abstract: Efficient Electrochemical N-Glycosylation of Silylated Pyrimidines with Protected Arylthioriboses in the Presence of a Catalytic Amount of NBS or Br2.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
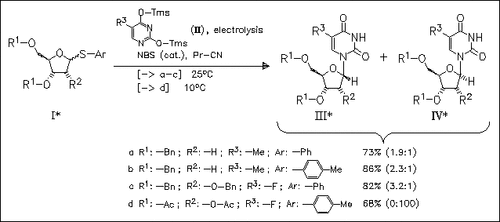
Electrolysis of silylated pyrimidines (II) with protected arylthioribose (I) in the presence of a catalytic amount of NBS or Br2 as mediator gives the corresponding nucleosides in good yields. The stereoselectivity of this glycosylation is similar to that of chemical glycosylation using a stoichiometric amount of NBS.