ChemInform Abstract: Regioselective N1-Alkylation of Guanosine Derivatives Protected at N2 by an N,N-Dialkyl Amidine Group.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
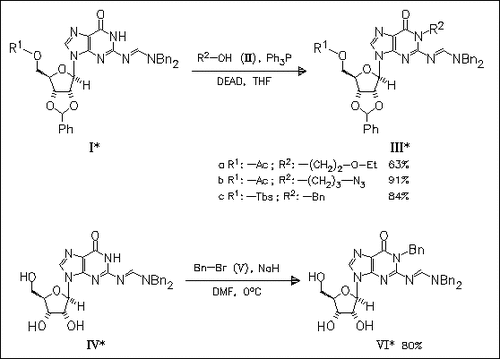
Under Mitsunobu conditions or in the presence of electrophilic alkylating reagents, alkylation of guanosine derivatives (I) and (IV), protected by an N,N-dialkyl amidine at the exocyclic amino group, occurs selectively on nitrogen N1 of the purine base.